Evolution and Ecology of Invasive Plant Species
The Biogeography of Invasive Plant-Soil Feedbacks and Plant-Pathogen Interactions
Project Overview
There is an extensive body of literature on the negative effects of soil pathogens and the positive effects of root symbionts on plant demography, community structure, food-web dynamics, nutrient cycling, etc. Ecologists have begun to recognize that plant-soil feedbacks can play a critical role in range expansion and species invasions across continents. The lack of host-specific soil-borne pathogens in the introduced range can facilitate the establishment and spread of plant species in the introduced range. Alternatively, successful establishment may be facilitated if the introduced plant species accumulates generalist soil pathogens that are more harmful to the native species, it possesses inhibitory allelochemicals that are novel in the introduced range, it quickly forms associations with beneficial soil organisms in the introduced range, or it suppresses mutualists associated with the native species. These studies support the notion that invasive plants are successful because they experience less negative (from pathogens) and/or more positive (from mutualists) feedbacks from soil biota in their invasive rather than native range.
A number of studies have shown that introduced species that are closely related to native species are less likely to successfully invade because they are more likely to gain negative interactions in their introduced range (e.g., Callaway et al. 2004). In some cases, however, phylogenetic similarity promotes invasion success, particularly if mutualists are involved (Brandt et al. 2009). Residence time in the introduced range and co-evolutionary history with soil microbes may also be important to the long-term success of invasive plant species (Richardson et al. 2000). For example, following establishment, invasive plants may accumulate new pathogens (or mutualists) or evolve stronger negative (or positive) interactions with them over time (Callaway et al. 2011). A hypothesis that emerges from these ideas is that microbial richness and the strength of positive and/or negative plant-soil feedbacks for invasive species should increase with residence time (likely asymptotically). By extension, we can hypothesize that for a range-expanding invader, plant-soil feedbacks are weakest at the leading edge of the invasion (see H1, Box 1). This hypothesis has never been tested as very few studies have considered invasive species interactions in a temporal context.
Providing a biogeographic context to the study of species invasions, particularly with regard to interactions between invasive plant species and members of the invaded community, is a new direction in the field of invasion biology (e.g. Cronin et al. 2015). Although there are a few studies that suggest soil microbe communities or their byproducts vary along a large-scale environmental gradient (e.g., latitude; Li et al. 2014), we know almost nothing about how plant-soil feedbacks vary spatially. Many of our best known invasive plant species have undergone continent-wide invasions, resulting in not only a latitudinal gradient but also a gradient in residence time (3, 30). A well known ecological paradigm suggests that species richness and the strength of species interactions should be greatest at low relative to high latitudes (Schemske et al. 2009). Although support for latitudinal gradients in species interactions is mixed, their occurrence is not uncommon. In keeping with this paradigm, H2 represents a plausible hypothesis regarding soil microbes (Box 1). If the microbial community or plant-soil feedbacks vary with latitude (H2) and/or location within the invasion range (H1), we hypothesize that this will contribute to spatial variation in invasion success or invader performance across the invasion range (hypothesis H3; Box 1).
Box 1: Biogeographic Hypotheses
- H1: The strength of plant-soil feedbacks is greatest near the invasion origin and decreases toward the invasion front.
- H2: Soil microbe diversity and feedback strength decreases with increasing latitude.
- H3: Biogeographic variation in plant-soil microbe interactions corresponds to variation invasive plant success/performance.

We are addressing the overarching hypotheses in Box 1 using European invasive genotypes of common reed, Phragmites australis, that were introduced into North America (NA) ca. 150 years ago and then spread westward across the whole continent (see Photo 1). A unique feature of this system is that native genotypes of P. australis are also widely scattered throughout NA and often co-occur with invasive genotypes (see Photo 1). We have a number of objectives, only some of which are currently under investigation.
(1) Survey soil fungal and bacterial diversity and community structure in the rhizosphere of invasive and native P. australis along latitudinal transects on the East Coast, Midwest and West Coast of NA, representing the longest to the shortest residence times of invasive P. australis (addressing H1 and H2).
(2) In common gardens, assess the net effect of soil microbes on native and invasive P. australis growth, defense, fitness and palatability to herbivore traits for plants from all over NA (H1 and H2).
(3) A greenhouse experiment was conducted to assess whether microbes mediate competitive interactions involving native and invasive P. australis (Allen et al. 2018).
(4) To better understand the mechanisms underlying P. australis-soil feedback, conduct a greenhouse experiment that partitions the effects of bacterial and fungal components of the microbe community on soil chemistry and P. australis traits.
(5) We will combine data from the previous 4 objectives with prior data collected that we collected on herbivory, and use structural equation models to assess the relative importance of below-ground and above-ground processes in affecting P. australis invasiveness and performance (H3).
This research project is being conducted in collaboration with Laura Meyerson at the University of Rhode Island and my former PhD student, Warwick Allen (Photo 2), now a postdoc at Lincoln University in New Zealand. Warwick was awarded a NSF Doctoral Dissertation Grant to pursue research related to Objective 3.

Community composition and impact of foliar and soil fungi
The main objective of this project is to quantify the community composition and impact of foliar and soil fungi among native, invasive and naturalized genotypes of P. australis. This project is led by Warwick Allen in collaboration with Meredith Blackwell, Professor Emeritus at LSU and world renowned fungal biologist. Using field surveys and a greenhouse experiment, we aim to test the following specific predictions: 1) exotic genotypes of P. australis have lower foliar and soil fungi diversity than native genotypes; and 2) exotic genotypes of P. australis will be less impacted by foliar and soil pathogens than native genotypes.
We conducted a survey of 35 field sites spanning 17° latitude from South Florida to Maine, with the aim of examining how microbe communities and pathogen damage vary geographically and among P. australis genotypes. Damage severity of pathogenic fungi was quantified by estimating the percent of area infected from randomly-selected diseased leaves per site. Diseased tissue was collected and fungal pathogens and endophytes were cultured on agar (Photo 3). Additionally, representative samples of diseased leaves, root segments, and rhizosphere soil were collected from each site. These leaf and root samples were surface-sterilized and all samples were transferred to cryovials containing RNALater (a RNA stabilization and storage solution) and stored in a -80 °C freezer. These samples will ultimately be used for genomic analyses of microbial communities. Results from this analysis are pending.
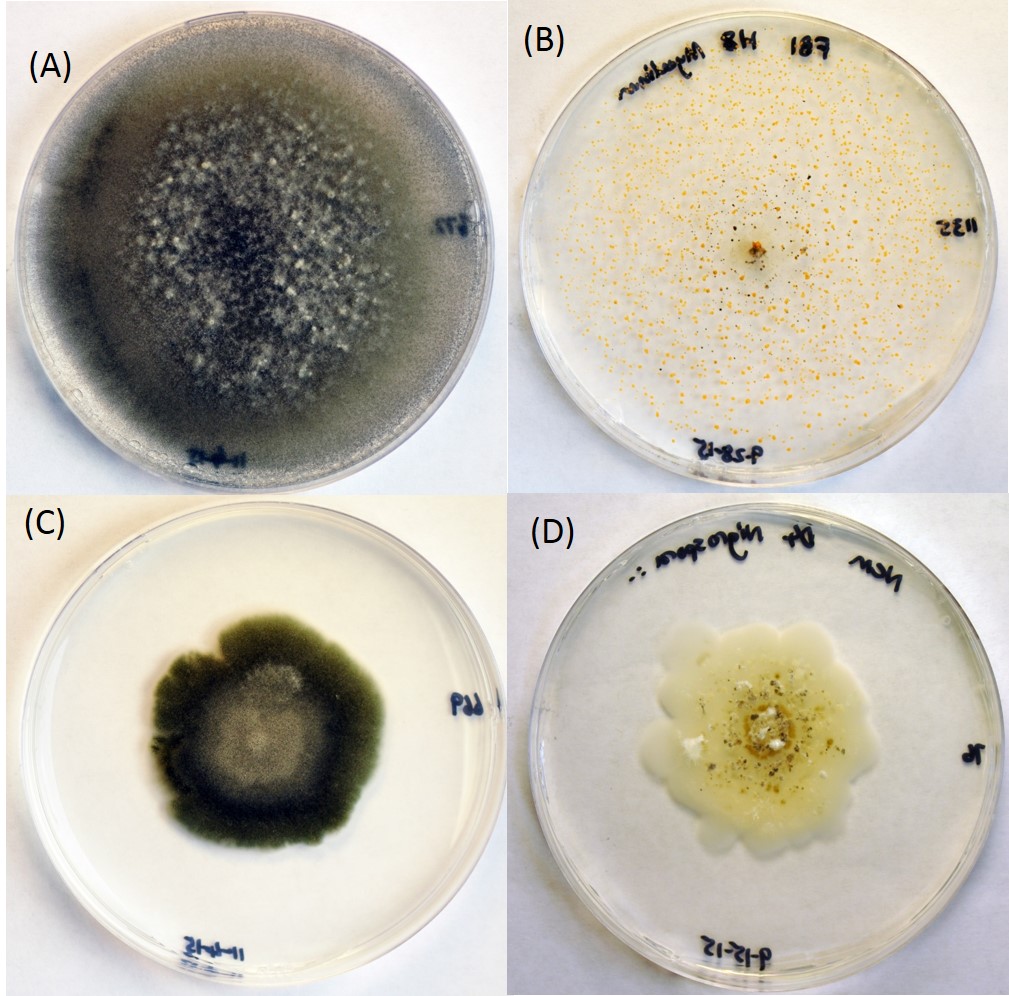
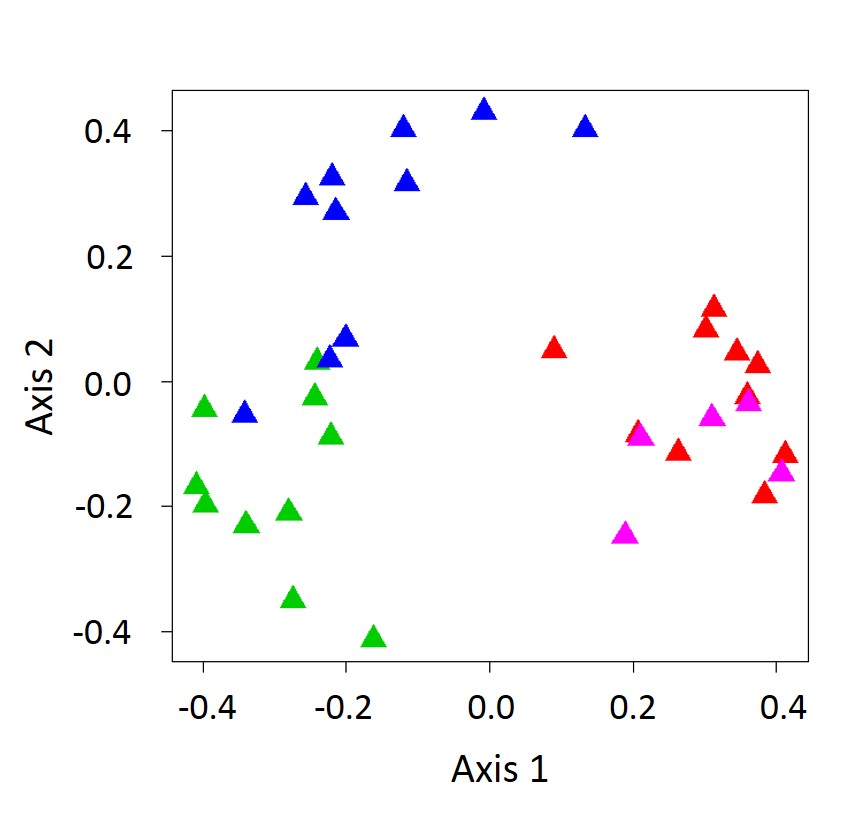
Based on Warwick’s survey of along the Atlantic and Gulf Coasts of the United States, he cultured 168 morphospecies (Photo 3). Based on a PCoA ordination plot, foliar fungi communities differ considerably among native, Gulf, European invasive (haplotype M), and Delta (another introduced genotype from Europe that is common in Louisiana) lineages (Figure 1).
Phragmites-soil feedbacks: Common-garden experiment
It is currently unknown how intraspecific variation in plant soil feedbacks (PSFs) interacts with the environment (e.g., nutrient availability) to influence competition between native and invasive plants. With Warwick Allen as the lead PI, we conducted a fully crossed multi-factor greenhouse experiment (Photo 4) to determine the effects of Phragmites australis rhizosphere soil biota, interspecific competition, and nutrient availability on biomass of replicate populations from one native and two invasive lineages of common reed (P. australis) and a single lineage of native smooth cordgrass (Spartina alterniflora). Harmful soil biota consistently dominated PSFs involving all three P. australis lineages, reducing biomass by 10%. Indirect PSFs (i.e., soil biota spillover) from the two invasive P. australis lineages reduced S. alterniflora biomass by 7%, whereas PSFs from the native P. australis lineage increased S. alterniflora biomass by 6%. Interestingly, interspecific competition and PSFs interacted to weaken their respective impacts on S. alterniflora, whereas they exerted synergistic negative effects on P. australis. Phragmites australis soil biota decreased S. alterniflora biomass when grown alone (i.e., a soil legacy), but increased S. alterniflora biomass when grown with P. australis, suggesting that P. australis recruits harmful generalist soil biota or facilitates S. alterniflora via spillover (i.e., apparent mutualism). Soil biota also reduced interspecific competition impacts on S. alterniflora, although it remained competitively inferior to P. australis across all treatments. Competitive interactions and responses to nutrients did not differ among P. australis lineages, indicating that interspecific competition and nutrient deposition may not be key drivers of P. australis invasion in North America. Although soil biota, interspecific competition, and nutrient availability appear to have no direct impact on the success of invasive P. australis lineages in North America, intraspecific lineage variation in indirect spillover and soil legacies from P. australis occur and may have important implications for co-occurring native species and restoration of invaded habitats. Our study integrates multiple factors linked to plant invasions, highlighting that indirect interactions are likely commonplace in influencing plant community dynamics and invasion success and impacts. This work was published in Ecology in 2018 (Allen et al. 2018).






