Roseau cane (Phragmites australis) Dieback
in the Mississippi River Delta
The Phragmites metabolome and how it varies among lineages, changes in response to environmental stressors, and is related to plant resistance/tolerance to herbivory and dieback symptoms
Plants respond to and defend against climatic stressors and biological invasions through changes in their phytochemical metabolome, yet ecologists have historically focused on a few targeted metabolites when assessing plant responses to stressors. Through the efforts of two postdoctoral associates in my laboratory, Ana Salgado and Andrea Glassmire, we investigated the metabolomic diversity of Phragmites and how it changed in response to different environmental stressors.
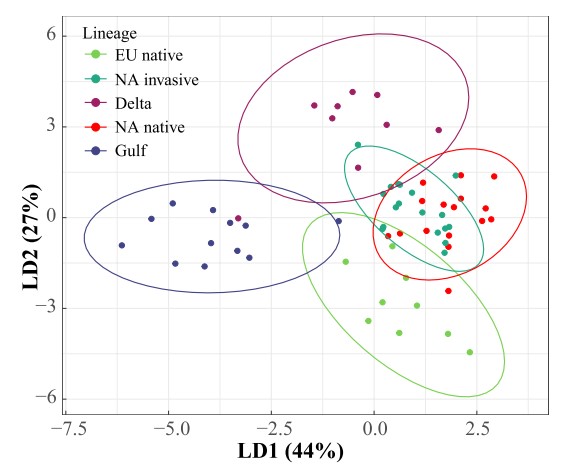
In the study by Salgado et al. (2023), we conducted an untargeted lipidomic and metabolomic analysis of five phylogeographic and ecologically distinct lineages of P. australis: European native, North American invasive, North American native, Gulf, and Delta. We found that lineages had unique phytochemical fingerprints, although there was overlap between the North American invasive and North American native lineages (Fig. 1). We also found that divergence in phytochemical diversity was driven by compound evenness rather than metabolite richness. Interestingly, the North American invasive lineage had greater chemical evenness than the Delta and Gulf lineages but lower evenness than the North American native lineage. Our results suggest that metabolomic evenness may represent a critical functional trait within a plant species. Its role in invasion success, resistance to herbivory, and large-scale die-off events common to this and other plant species remain to be investigated.
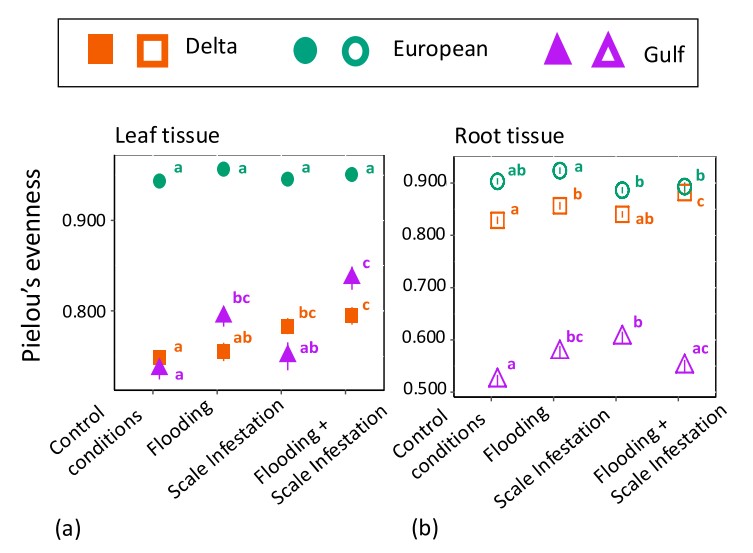
Using the methodology pioneered in the Salgado et al. (2023) paper, Andrea Glassmire conducted a study to explore how inundation and scale insects could affect the metabolomic diversity of the three main Louisiana lineages of Phragmites. Results indicate that metabolomic responses are highly context specific. Metabolic diversity, measured as Pielou’s evenness, varied with lineage, tissue type, stressor type, and the presence of multiple stressors (Fig. 2). Notably, the non-native lineage displayed high chemical evenness, while the other two lineages showed stressor-dependent responses. The 10 most informative chemical features identified by a machine learning model showed less than 1% overlap with known metabolites linked to water and herbivory stress, underscoring gaps in our understanding of plant responses to environmental stressors. Our metabolomic approach offers a valuable tool for identifying candidate plant genotypes for wetland restoration.
In collaboration with Laura Meyerson (https://web.uri.edu/nrs/meet/laura-a-meyerson/) at the University of Rhode Island, Tom Mozdzer (https://www.brynmawr.edu/inside/people/thomas-mozdzer) at Bryn Mawr University and Andrea Glassmire, we are also examining how elevated CO2 and fertilization are affecting the invasive Phragmites metabolome and how these phytochemical changes in the plant have cascading effects throughout the marsh community. This work was conducted in cooperation with the Smithsonian’s Environmental Research Center (https://serc.si.edu/) at the Global Change Research Wetland (https://serc.si.edu/gcrew). In this 70 ha marsh on the Chesapeake Bay, open-top chambers were set up on the leading edge of a Phragmites invasion in 2011 and were subjected to one of four treatments: elevated CO2, elevated nitrogen, elevated CO2 + elevated nitrogen and an ambient control (Fig. 3). In 2022, we collected tissue samples from all plant species, soil samples and volatile organic compounds (VOCs). We are in the process of determining how these climate-change variables affect metabolomic α diversity (i.e., diversity within a plant) and β diversity (i.e., among Phragmites plants and all plant species), soil-microbial diversity, VOC diversity and herbivory.

Finally, in 2024, Andrea Glassmire, Jonathan Richards (https://www.lsu.edu/agriculture/ppcp/people/profile/jonathan-richards.php) and I conducted an experiment to explore the effects of drought and scale herbivores on Phragmites metabolome and transcriptome. We used Phragmites source populations derived from the coast-wide survey in 2022 in which we quantified numerous metrics associated with Phragmites lineage, genotype, biomass, microbial community and herbivores; as well as other traits associated with the site (elevation, soil chemistry and, plant diversity). One of our goals with this study was to identify source populations of Phragmites that could be useful for restoration; i.e., plants that grow vigorously and are resistant/tolerant to herbivory, drought stress and dieback symptoms. We set up a common-garden experiment using 48 Phragmites populations split evenly between Delta and EU (Fig. 4). We subjected one half of the plants to a drought treatment and the other one half to a well-watered treatment. Subsequently, we released scale insects into the experiment to allow them to attack plants. Undergraduate student Emma Klenke’s honor’s thesis focuses on the effects of these stressors on plant biomass. Andrea Glassmire is conducting the metabolomic and VOC analyses and Jonathan Richards is going to conduct the transcriptomics analyses. Ultimately, we plan to examine the link between metabolomic and transcriptomic changes in the plants and how that varies among Louisiana populations of Phragmites.






