Roseau cane (Phragmites australis) Dieback
in the Mississippi River Delta
The role of soil microbes in mediating Phragmites-herbivore-natural enemy interactions
Starting with the work of my former Ph.D. student, Warwick Allen, my lab has been interested in the role that foliar and soil microbes play in mediating Phragmites interactions with other plants and their herbivores and natural enemies. Warwick’s excellent Ecology paper (Allen et al. 2018) demonstrated that soil microbes have a net negative impact on Phragmites growth and biomass and that those microbes influence competitive interaction between Phragmites and Spartina alterniflora. Phragmites soil biota decreased S. alterniflora biomass when grown alone, suggesting there is a soil legacy of Phragmites. Interestingly, plant-soil feedbacks varied with Phragmites lineage. You can read more about this in Warwick’s paper.
More recently, Joseph Johnston became interested in the role of soil microbes in the dieback of Phragmites in the MRD. Joe observed that frequently after a Phragmites dieoff, elephant ear or taro (Colocasia esculenta) colonized that open space. In fact, taro has become more prevalent during this dieback event in the MRD (Elsey-Quirk et al. 2024). Joe questioned whether it was possible for Phragmites to successfully re-establish in former dieback sites that were presently occupied by taro. He asked the question, can Phragmites outcompete taro? And, is it possible that taro-soil microbes play a role in that competitive interaction? With Joe as the lead, we conducted a common-garden experiment to investigate the competitive interactions among taro and two Phragmites lineages, Delta and EU, and the role of taro soil microbes in mediating those interactions.
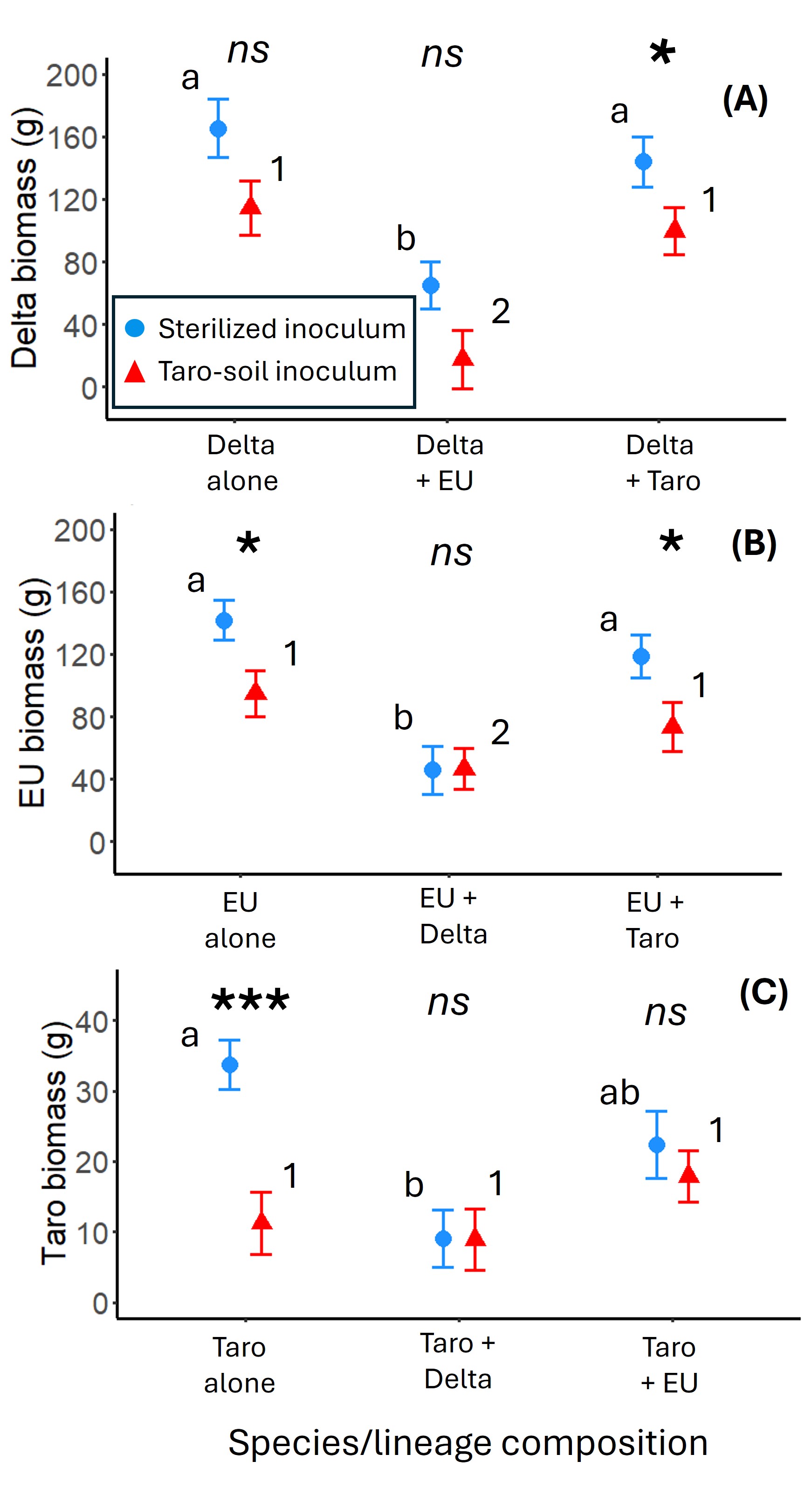
Plant types were grown alone or together and crossed with a microbial treatment (sterilized or live taro-soil biota) (Fig. 13). After one growing season, we measured plant above- and belowground biomass and collected soil samples for later determination of the soil-microbial community.

The results of this experiment formed the basis for Joe’s M.S. thesis and manuscript that is currently in review for publication (Johnston et al. in review). Joe found that in the absence of taro microbes, Delta and EU had equivalently strong negative effects on each other’s biomass and the biomass of taro; whereas, taro had only a small negative effect on the biomass of both lineages of Phragmites (Fig. 2). However, taro-soil microbes had a strong legacy effect, reducing Delta and EU biomasses by 30-33% and taro by 66% when plants were grown without a competitor. Interestingly, when Delta and EU were in competition, the soil legacy resulted in EU being a better competitor – total biomass of Delta and EU were reduced by 90% and 68%, respectively, in comparison to no-microbe/no-competitor controls. Overall, the effects of competition and the taro-soil legacy on Delta and EU were determined to be additive. In contrast, taro microbes and Phragmites competitors acted antagonistically to affect taro’s biomass, resulting in only a 67% (with Delta) or 48% (with EU) reduction in taro biomass relative to experimental controls. We conclude that restoration of Phragmites in the MRD may require manipulating the soil community to mitigate taro-legacy effects and choosing the Phragmites lineage that is the best competitor in the presence of plant-soil feedbacks.